At STIM Canada we believe that the needs of our customers are paramount. As a result we have developed a Quality Management System that ensures the products and services we provide will be of the quality demanded by the customers.
We recognize the contribution required from all employees to realize this quality goal and value them as our strongest asset. Quality is the responsibility of all. Also, we recognise the crucial influence our suppliers have and shall ensure that we will develop mutually beneficial relationships.
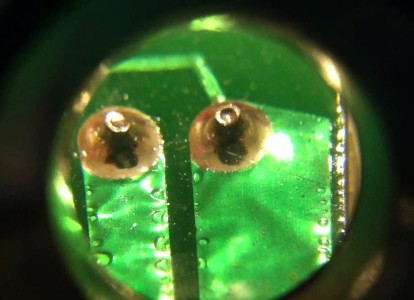 STIM Canada is able to measure and track defects throughout production. This includes rapidly and accurately identifying flaws, reasons for failures, and error trends in order to ensure product quality and overall yield — for example, whether or not it is a specific vendor, a certain material or an individual part of the process that is causing poor results.
STIM Canada is able to measure and track defects throughout production. This includes rapidly and accurately identifying flaws, reasons for failures, and error trends in order to ensure product quality and overall yield — for example, whether or not it is a specific vendor, a certain material or an individual part of the process that is causing poor results.
Our ISO 9001 and ISO 13485 (Medical) accreditations are complimented by many other recognised electronic manufacturing standards and endorsements to ensure correct specifications are used in the manufacturing process.
At STIM, each step of the assembly process is noted electronically and sequenced in the order that it needs to take place. Inspection points are set at every stage and first article checks are made to ensure the process flows error-free. Every operator has a unique identifier so that the workflow is traceable to the person who completed the task. This provides complete traceability for every step of the operation from when it enters STIM to the finished product.
